How to Calculate Concentration of Ion in Solution if You Know Its Conc and Volume Dissolved
4.4: Measuring Concentrations of Solutions
- Page ID
- 52102
Learning Objectives
- Ascertain Molarity
- Differentiate between solute, solvent, and solution
- Calculate the molar concentration for solutes
- Outline the steps to brand a solution of a desired concentration from a solid or aqueous solute
- Summate the concentration of ions in a soluble ionic compound
- Perform stoichiometric calculations involving aqueous solutes
- Calculate the concentration of unknown solutes
Introduction
Upwards to this point we have used stoichiometry to "count" atoms, molecules and ions by measuring mass of pure substances and using molar masses to calculate the number of chemical entities (moles). Many of the reactions we take studied involve solutions, where nosotros are interested in a solute that is dissolved in a solvent, and we can not mensurate the mass of the solute contained of the solvent. We besides need to realize that many chemical reactions require the reactants to be mobile, where they tin crash-land into each other, and this can occur when they are dissolved in a solvent. So information technology is very important that nosotros can count chemical entities when the entity of involvement is a solute dissolved in a solvent, and in which case we measure out the mass of book of the solution as a whole (solvent plus all solutes) of which volume is typically the easiest to measure.
Concentration of a Solute
There are two bones ways of reporting the concentration of a solute in a solvent, by reporting the mass of solute in a given volume, or the number of moles of solute in a given volume. These are effectively conversion factors that define the equivalent mass or moles of a solute to the volume of the solution.
- Mass Concentration: has typical units of g/L \[\frac{mass\: solute}{vol \: solution}=\frac{k(one thousand))}{V(50))}\]
- Mole Concentration (Molarity): has units of mol/L or M \[\frac{moles\: solute}{vol \: solution}=\frac{n(moles))}{V(L))} = M(mol/L)\]
So a 3.0M solution of sucrose has 3.0 mole of sucrose per liter
How do nosotros catechumen between mass and mole based concentration?
Simply by multiplying or dividing past the tooth mass (g/mol). If you know the grand/L, yous only divide by the molar mass, and if you know the moles/50 and want the mass/Fifty, you multiply by the molar mass (simply utilize the molar mass every bit an equivalence statement).
How do nosotros "count" solute molecules and ions?
By measuring the book and knowing the molarity. This is analagous to measuring the mass and knowing the tooth mass for a pure substance.
\[n(moles)=M(\frac{mol}{L})(5(L)) \\ n=MV\]
This can be contrasted to how nosotros "count" chemical entities that are pure solids.
\[n(moles)=\frac{m(g)}{molar \; mass (\frac{one thousand}{mol})} \\ n=\frac{grand}{fw}\]
Making a Solution with a Solid Solute.
Step i: Calculate Mass of solute needed for desired book
Pace 2: Quantitatively Transfer Mass to Volumetric Flask
Footstep 3: Dilute to volume with solvent, ensuring that all of the solid has dissolved.
Making 500.0 mL of 0.500 1000 Copper(Ii)Sulfate
Step 1: Calculate Mass CuSO4(s) needed.
\[0.5000L\left (\frac{0.500molCuSO_{4}}{L} \right )\left ( \frac{159.6gCuSO_{4}}{mol} \right )=39.9gCuSO_{iv}\]
Pace 2: Weight 39.9g CuSOiv(s) and quantitatively transfer to 500 mL graduated cylinder (which is calibrated to 500.0 mL), being certain all the salt is transferred.
Footstep iii: Fill half style and mix, then dilute to volume. Make sure all the solute is dissolved, and recheck that solution level is at bottom of meniscus.
Practice \(\PageIndex{1}\)
What is the molarity of a solution made when 66.two grand of C 6 H 12 O 6 are dissolved to brand 235 mL of solution?
- Answer
-
1.57 1000 C six H 12 O vi
Why can't you lot just add 500.0mL of water to 39.9 g CuSOiv to make 500.0 mL of 0.500M solution?
Kickoff, this is not taking into account the volume of the solute, and 2d, there is ordinarily a change in volume when you dissolve a solute into a solvent, and in the instance of ionic compounds this is a wrinkle.
Exercise \(\PageIndex{2}\)
Using concentration as a conversion factor, perform the following adding.
A) How many liters of 0.0444 M CH ii O are needed to obtain 0.0773 mol of CH ii O?
B) What mass of solute is present in 1.08 L of 0.0578 M H two So iv ?
C) What volume of ane.50 One thousand HCl solution contains 10.0 g of hydrogen chloride?
- Answer
-
A) one.74 L
B) 6.12 g
C) 183 mL or 0.183L
Ion Concentrations in Solution
When an ionic compound dissolves it breaks upward into its ions.
(NH4)2Cr2O7(aq) --> 2NH4 +(aq) + Cr2O7(aq)
Dissolving 252.07 grams of ammonium dichromate (fw = 252.07 g/mol) results in a solution that is 1M ammonium dichromate, but when an ionic salt dissolves, it breaks up into ions, and so what you really have is a solution that is two K ammonium ion (NH4 +) and 1 M in dichromate ion (CriiO7 +2)
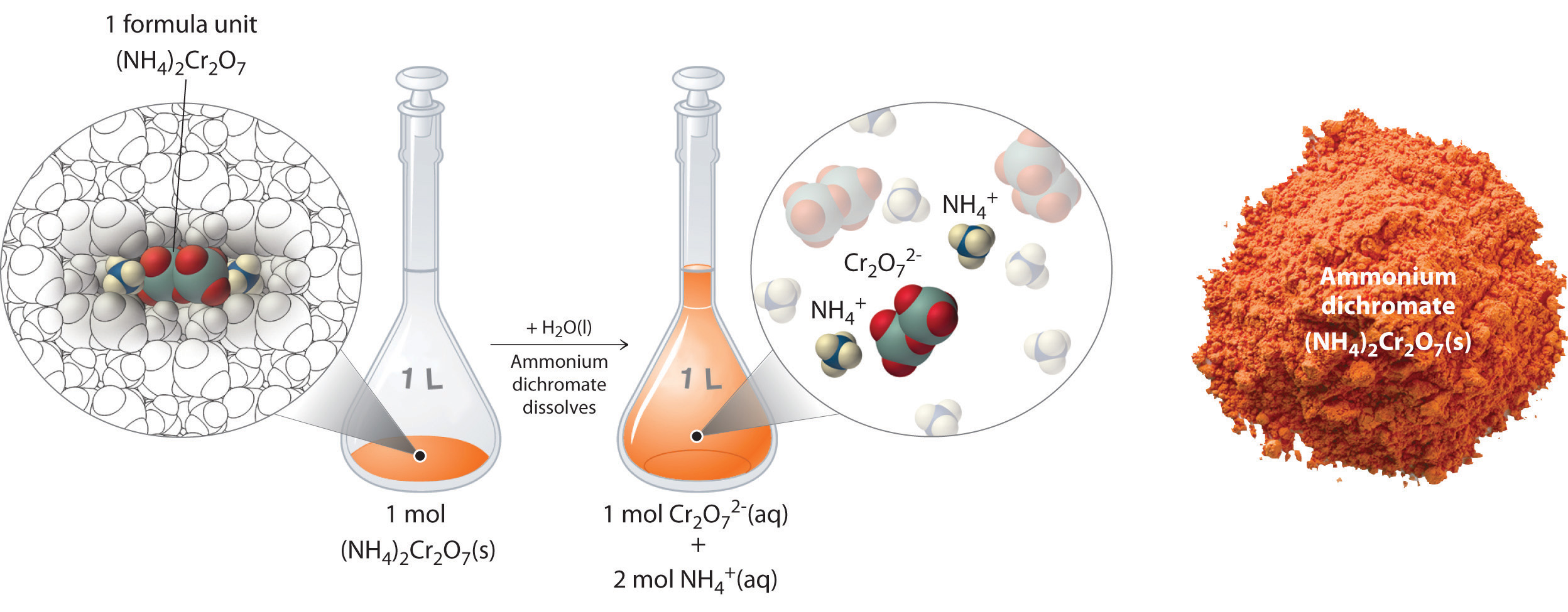
Dissolution of 1 mol of an Ionic Chemical compound. In this case, dissolving 1 mol of (NH4)2Cr2Ovii produces a solution that contains i mol of Cr2O7 2− ions and 2 mol of NH4 + ions. (Water molecules are omitted from a molecular view of the solution for clarity.)
Example \(\PageIndex{1}\) Solution Concentration of Iron(2)chloride
Water is added to ii.16 1000 of the ionic chemical compound ferrous chloride to make a solution with a full volume of 100.0 mL. Limited the concentration of the salt solution, and that of its ions.
1. What is the salt concentration?
two. What are the ion concentrations?
Solution
Video \(\PageIndex{1}\) goes over this calculation.
Ans: 0.170M FeCl2(aq)
0.170M Fe+ii(aq)
0.340M Cl-(aq)
Yeah, in that location are three answers! (that of the salt, and of the ions)
Video \(\PageIndex{one}\): 3'36" YouTube computing the concentration of a Ferrous Chloride in example \(\PageIndex{1}\).
Practice \(\PageIndex{iii}\)
You have a 1.50 K solution of Na2CO3. What is the concentration of:
A) sodium ions?
B) carbonate ions?
C) full ions?
- Answer
-
A) [Na+] = iii.00 One thousand
B) [CO3 -ii]= 1.50 M
C) [Na + ] + [CO3 -two] = four.50 M ; or yous can choose think for every 1 mole of Na2CO3, there are three moles of ions (identify of the ions is irrelevant).
Preparing Solutions with Stock Concentrated Solutions (Dilution).
Many stockroom reagents come as full-bodied solutions that can easily be diluted to a desired concentration past adding solvent. Adding a solvent does non change the moles of solute (n), then
northwardinitial = nfinal
northi = nf
MiFivei = MfFivef
Step 1: Calculate initial volume of stock
Step ii: Transfer to volumetric flask with a volumetric pipette
Step 3: Dilute to Volume
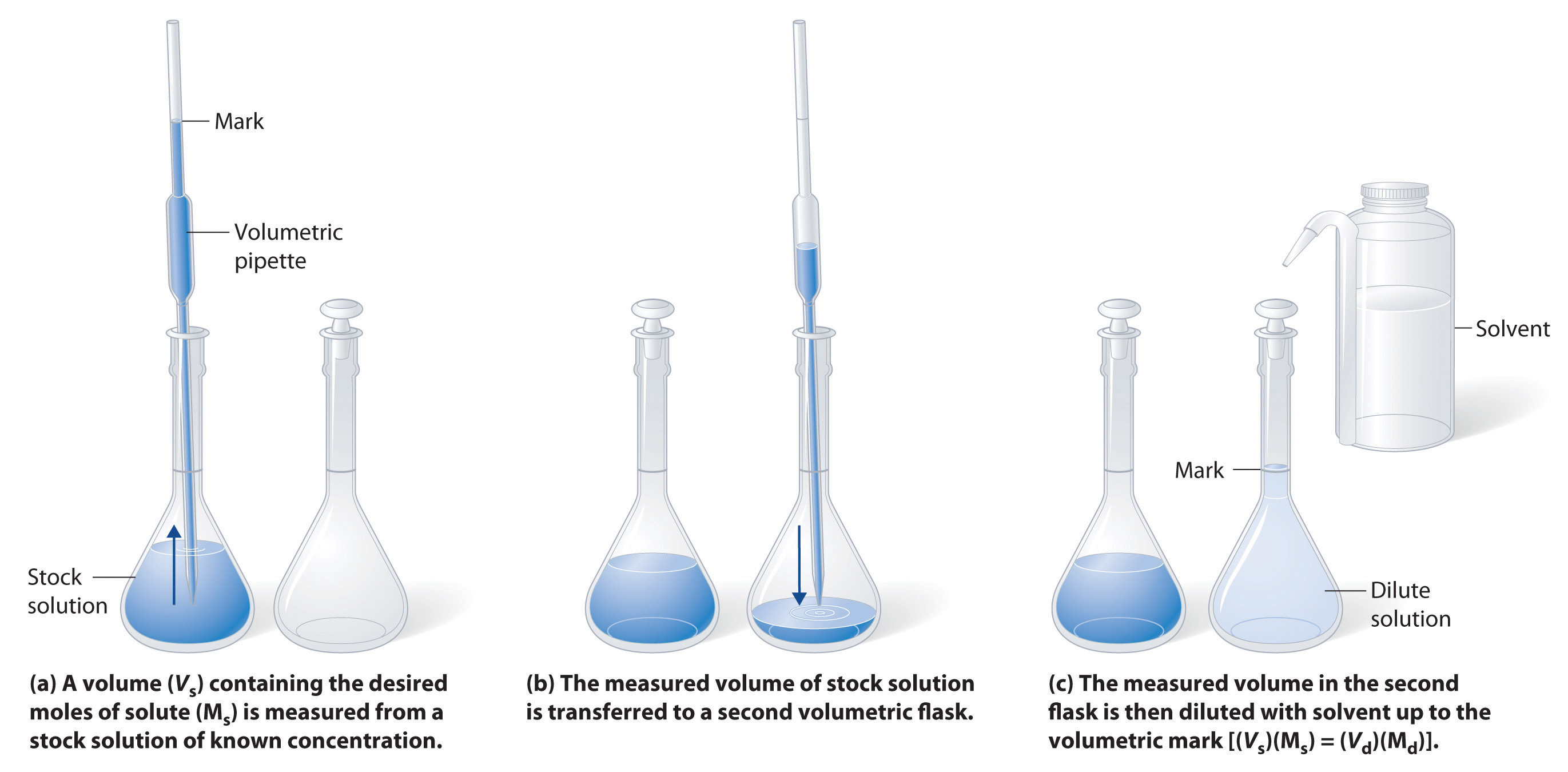
Example \(\PageIndex{2}\)
How practice y'all brand 500.0 ml of 0.70M HCl solution from stock 11.6 G HCl?
Solution
Step ane: Calculate initial volume of 11.6 M hydrochloric acid needed.
\[M_{i}V_{i}=M_{F}V_{F}\]
\[V_{i}=V_{f}\left ( \frac{M_{f}}{M_{i}} \correct )=500.0mL\left ( \frac{0.70M}{xi.6M} \right )=30.2mL\]
Step 2: Quantitatively transfer this volume to a 500 mL volumetric flask (this has 4 meaning Figures, that is, information technology is calibrated to 500.0 mL)
Step 3: Dilute with water to marker
Exercise \(\PageIndex{4}\)
What is the concentration of an HCl solution if 598 mL of 0.778 M HCl is diluted to one.00 L?
- Reply
-
e. five.23 * 102 mL
\(M_{i}*V_{i}=M_{f}*V_{f}\)
\(M_{f}=\frac{\left (M_{i}*V_{i} \right )}{M_{f}}=\frac{ 0.778 Chiliad\left ( 0.598L \right )}{one.00 L}= 0.465 1000 \)
Contributors and Attributions
Robert East. Belford (University of Arkansas Little Stone; Department of Chemistry). The breadth, depth and veracity of this work is the responsibleness of Robert E. Belford, rebelford@ualr.edu. Yous should contact him if you lot accept any concerns. This material has both original contributions, and content congenital upon prior contributions of the LibreTexts Customs and other resources, including just non limited to:
- November Palmer & Ronia Kattoum (UALR)
- bearding
Source: https://chem.libretexts.org/Courses/University_of_Arkansas_Little_Rock/Chem_1402%3A_General_Chemistry_1_(Belford)/Text/4%3A_Stoichiometry%3A_Quantitative_Information_about_Chemical_Reactions/4.4%3A_Measuring_Concentrations_of_Solutions
0 Response to "How to Calculate Concentration of Ion in Solution if You Know Its Conc and Volume Dissolved"
Post a Comment